By Eric Sideman, Ph.D.
Lead is an element that is lumped with a group called heavy metals because of their similar chemical characteristics. Some of these metals are necessary nutrients in small amounts for plants and/or animals, but as a general rule, each becomes toxic at some concentration. Copper and zinc are essential in very small amounts for normal health but in larger amounts become toxic for both plants and animals. Most of the heavy metals are just plain toxic because they interfere with the biochemical activity in cells.
All of these metals exist in all soils at some “normal background level,” which does not pose a risk to plants, animals or people. However, human habitation and activity tends to elevate the concentrations of these metals. When the concentration goes above “normal,” a site is said to be contaminated with the metal in question. When the concentration goes so high that it becomes a risk for plant or animal health, the site is said to be polluted.
Some metals (chromium, copper, nickel and zinc) become toxic to plants at high concentrations but do not pose a risk to animals because the uptake of these metals by plants will kill the plant before it could be eaten or harvested for food. Cadmium, mercury and lead are greater concerns for humans because plants can tolerate relatively higher concentrations of these and accumulate enough metal to pose a risk before effects are seen in the plant.
Lead is the most common heavy metal contaminant of soil. Natural background levels of lead range from <1 to 40 ppm (parts per million), depending on the parent material of the soil, and soil amendments can add lead to the soil (Table I), but pollution is the more common reason that soils are higher in lead than would be predicted.
Common sources of pollution include pesticides used in orchards in the earlier part of this century, scrap metal heaps, wastes from mines, and paint chipping off of buildings. Paint is a common source of soil lead, but forms a halo of contaminated soil around buildings and does not affect a large area. The largest source of pollution has come from combustion of gasoline when it contained a lead-based antiknock compound. That compound is no longer in gasoline, but it accounted for 80% of the lead in the atmosphere. Half of the combusted lead fell within 100 meters of the road. The rest ended up floating in the air and is now distributed widely. This is evident in the dramatic increase in lead concentration in snow in cores from Greenland from the 1940s to the 1980s.
Lead owes its toxicity to its interaction with sulfhydryl groups of protein molecules. It interferes with functions associated with cellular membranes, so kidneys and nervous systems are major sites of damage. Membrane functions that are most affected are absorption of sugar and phosphate by kidney cells and energy-linked transport of sodium and potassium in red blood cells. Considerable evidence shows that the disturbance of kidney and brain functions by lead may reflect its influence on mitochondrial ATP synthesis, which provides the basis for all energy requiring reactions in cells.
Lead in animals leads to anemia, which means there is a deficiency in the red pigment of blood, hemoglobin. This deficiency is due to three effects of lead: 1) lead toxicity leads to production of excessively fragile red blood cells; 2) lead interferes with the synthesis of the hemoglobin, a reaction catalyzed by an enzyme that requires a sulfhydryl group for activity; and 3) the incorporation of iron into the heme ring is impaired by lead, thus iron tends to build up in the cells. No one knows lead has the same effect on heme synthesis in plant cells. Lead can be toxic to plants because it mimics the metallic properties of calcium and inhibits enzyme systems.
Lead in Soils
Once lead gets into the soil it is virtually a permanent resident. It is the least mobile of the heavy metals, which as a group are not mobile. Lead is associated in the soil with clay minerals, manganese oxide, iron and aluminum oxides, and organic matter. In some soils that are highly contaminated, it is associated with calcium carbonates or phosphates. Lead compounds are very stable in the soil, taking an estimated 200 years for a 10% decrease by leaching or degradation. Because lead is quickly bound in soil, airborne contamination is usually restricted to the top few centimeters of the soil profile. Only in acid soils does lead move through the soil profile. Liming soils greatly decreases the solubility of lead by forming the hydroxide, phosphate, and carbonate compounds. A high pH also promotes formation of lead/organic complexes, especially in soils with high organic matter levels.
Lead in Plants
Years ago concern about lead in soil was minimal because of the relative insolubility of adsorbed and precipitated compounds of the metal. Recent research has shown that the lead concentration of soils is directly correlated with lead concentration of plants, which indicates that the metal is taken up by plants.
Lead toxicity can reduce plant growth, but this is not generally seen in the field. Almost all detailed observations of lead toxicity in plants are restricted to water culture experiments, which are rarely applicable to plants growing in soil.
Lead is not a nutrient for plants, and its uptake is primarily passive. Uptake is reduced by factors that reduce its solubility, such as cold temperatures and high pH. Once taken up by plants from the soil, translocation of lead is usually greatly limited. Lead accumulates in the cell wall, which may well protect the plant from toxic effects. In maize plants, lead is first concentrated in cellular vesicles that fuse together to encase the lead deposits. These then are removed from the cell cytoplasm to outside the cell membrane to fuse with the cell wall.
In many plant species studied, only 3% of the lead taken up is translocated. Even over 500˛0 lbs/A of lead added to the soil resulted in only an increase of only a few parts per million of lead in barley tops, but exceptions exist and not all have been studied. Leaf crops such as lettuce efficiently concentrate lead in their tops. Eggplants can concentrate relatively large amounts in the fruit. Generally fruit crops have less than root crops and root crops less than leafy crops, but I wouldn’t eat by that rule out of a garden that showed lead contamination.
Lead on the surface of plants is often a greater concern than that taken up by the plants. Experiments with plants alongside highways showed 50 ppm in plants right on the side of the road and only 2 to 3 ppm 150 meters from the road. The highest concentrations were on the outer parts of the plants, while concentrations in tubers and roots were nearly normal (0.5 ppm).
Precautions
Lead in the soil threatens people in two ways. The first is by direct ingestion of lead-bearing soil. Children are at greatest risk because they commonly eat without washing there hands and commonly put their hands in their mouths while playing in the soil. Everyone is at risk from dust and particles clinging to vegetables that are not well washed. Remember, soil particles are always going to be on the surface of vegetables, having been kicked up by foot traffic in the garden, splashed from rain and sprinklers, and stirred up as dust during cultivation.
The second way lead is ingested is by eating vegetables that have taken it up excessively. This is usually minimal if the precautions listed below are taken. Bruce Hoskins from the Maine Soil Testing Laboratory said he has seen studies that show very minimal uptake at very high soil lead levels if care is taken to avoid contamination of above-ground tissue during the treatment application process. Disparities between these studies and others that show significant tissue content always beg the question of whether the “uptake” is actually insufficiently removed surface contamination. All this is rather moot if you consider that edible plant parts will be washed quickly, incompletely, or not at all in some cases. Whether the lead is internal or external makes no practical difference since it is all ingested anyway.
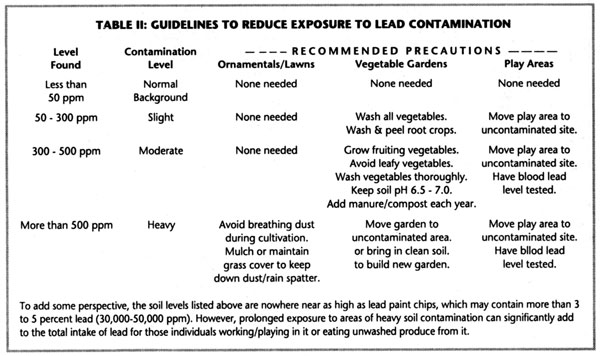
As part of the standard soil test done at the University of Maine, a screen for lead contamination is now conducted. Anything less than 50 ppm is considered a normal background level in Maine. Contamination levels are as follows:
• slight contamination 50 to 300 ppm
• moderate contamination 300 to 500
• heavy contamination >500
If the soil test for lead comes back moderate or high, then the lab sends an excellent sheet of guidelines about avoiding uptake by plants and lead poisoning. The sheet includes the following recommendations (see Table II):
Here are some pointers that I think are important if your test results come back moderate:
• Raise and maintain the organic matter level to around 10% if you can get there;
• Raise and maintain the pH from 6.5 to 7.0 (Do not over do it. Too much lime can limit the availability of nutrients for plants);
• If the pH is appropriate and the organic matter level is high enough, then surface dust and soil particles are a greater risk than uptake by plants;
• Surface contamination can be reduced with a mulch;
• Wash vegetables very well and peel root crops.
If your test comes back with a heavy contamination of lead (above 500 ppm), I suggest growing vegetables somewhere else.
About the author: Eric is MOFGA’s director of technical services. You can direct your questions about farming and gardening to him at the MOFGA office.
 |
 |
