Good summer conditions have left many of us forgiving and forgetting the cold and wet start to the growing season. However, some of the ramifications from the winter and spring are still showing themselves.
Vern Grubinger wrote a nice summary of mummyberry for highbush blueberries, that he allowed me to share here. It’s too late to stop this year’s infection, as the spread of the disease occurred in the rainy spring, but it’s important to notice how many mummies are present in your crop as you harvest this year, in order to know how much disease pressure may be present next spring to re-infect and start the disease cycle again.
In addition to the “usual” garlic problems to watch out for, you may have seen elsewhere that David Fuller, Eric Sideman and I are pondering the conditions leading to reports of multiple scapes being produced by single garlic plants. The growers that have reported the issue to us have seen between 5% and 50% of their crop affected, with each affected plant sending up two-to-five scapes, instead of the usual one. These later scapes are trapped within the leaf sheath layers of the “mother” plant. The best guess so far, as to the cause of these affected plants, is one or more stresses early on in the season triggering this year’s cloves to sprout and send out a scape. Standing water this spring may have played a role. If you had this issue, please let me know.
Mummyberry in Blueberries
Garlic Problems
MUMMYBERRY in Blueberries
By Vern Grubinger
There has been a lot of this disease around the region due to spring weather conditions that favored infections. Late frost may have exacerbated the susceptibility of shoots as well.
As you may know, mummyberry goes from the soil (spores released by little trumpet-like mushrooms around the time forsythia blooms) to shoots (‘primary’ infection, causing wilting dark leaves and stems) which then produce a different kind of spore that blows/goes on insects to blossoms and causes ‘secondary’ infection of fruit. The infected fruit (‘mummies’) drop to the mulch/soil and start the process over next spring. The mummyberries need a cold treatment to produce spores so are not infective this fall, based on what I have read.
At this point in the season, all of your ‘secondary’ fruit infection (shoot to blossoms) has already taken place for the year, so there is no benefit to spraying fungicides for this disease. Remove infected berries as best you can, before or after they drop to the ground and overwinter, to help reduce disease pressure next year.
Applying a thick layer of mulch in spring is key, to bury the little mushrooms. Raking the mulch in early spring can also help. I’ve been asked about laying down landscape fabric or other materials on top of mummies next spring, and it makes sense but I am not aware of evidence that it works.
Fungicides only work against mummy berry infection preventatively, whether shoot and/or fruit infections. There is usually different efficacy for shoot strike (primary) and fruit (secondary) infection among fungicides. If you decide to spray next year you should rotate at least two different FRAC group (mode of action) fungicides. See the table in the New England small fruit guide: https://ag.umass.edu/fruit/ne-small-fruit-management-guide/highbush-blueberries/diseases
Next spring, scout for the ‘trumpets’ and protect the shoots before they release spores (again, usually about when forsythia blooms). Conventional and organic growers then have several spray options.
Organically, you could alternate Regalia with either Double Nickel or Serenade plus spreader sticker (NuFilm). These (OMRI) materials have different modes of action so they suppress fungi differently. Alternate the sprays, re-applying at the shortest spray interval that the labels allow if you have had high pressure this year.
Good spray coverage is very important, use a spreader-sticker, and get a decent sprayer. Spray early or late, when pollinators are not active. Well-timed sprays, prior to leaf and then fruit infection will very be important to effectiveness of the sprays – just spraying randomly is not likely to be effective. Materials need to be reapplied after heavy rains.
This disease should be manageable (I have seen that!) by deploying all the cultural practices plus some well-timed sprays, but you do not want to let it get ahead of you. Re-mulching annually is very important!
Here are good fact sheets on mummyberry disease, with images of the symptoms:
- https://blogs.oregonstate.edu/mummyberry/files/2014/05/MUMMY-BERRY-FACT-SHEET.pdf
- https://www.canr.msu.edu/news/scouting_and_management_of_mummy_berry_in_blueberries
GARLIC PROBLEMS
It is time to harvest garlic, and it is time to identify any problems you may have. These are some of the common problems to watch out for now that you are harvesting, and for when buying and planting garlic seed this fall. Some of these are only transmitted by planting diseased cloves or by moving contaminated soil.
 |
| Damage from garlic bloat nematode |
GARLIC BLOAT NEMATODE (Ditylenchus dipsaci)
The stem and bulb nematode, also called the garlic bloat nematode, is a relatively new garlic pest in the Northeast, first appearing in New York in 2010 and now being found throughout the Northeast. It has been spread by infested garlic seed. The microscopic worms feed by piercing root and leaf cells with their stylet. Leaves of severely infected plants turn yellow and dry prematurely. Plants may be stunted. The roots may be missing and the basal plate may appear to have a dry rot similar to Fusarium basal plate rot.
The pest is favored by wet, cool conditions. Although the pest is not active in hot dry weather, such weather may exacerbate symptoms. The nematode survives freezing and hot weather in soil and plant debris. It can only move short distances on its own. It is primarily moved by growers either moving soil (on tools, boots, etc.), in moving water, in debris from garlic or onion, or most commonly in garlic bulbs used for seed.
The best way to avoid garlic bulb nematode is to use your own garlic for seed, IF it is not yet infested. Monitor for symptoms of infestation during the growing season and submit suspect plants to a diagnostic lab for confirmation. Garlic bloat nematode testing is now being performed by Dr. Alicyn Smart at the UMaine Plant Disease Diagnostic Lab, for a $20 fee for in-state samples. Sample submission information can be found here:
If it is determined that you do have the problem, DO NOT use your own garlic for seed. Even bulbs that show no symptoms may have low levels of infestation. Obviously, do not sell any garlic for seed from a potentially infested lot. Do not replant garlic in an infested field for at least four years. Other hosts include all alliums, celery, parsley, and salsify. Mustards, sorghum-sudan grass, and other bio-fumigant cover crops planted during the rotation period have been shown to reduce nematode populations in a field. These nematodes can survive in dry debris so carefully clean equipment and storage areas.
There are currently no materials that offer control.
 |
| Fusarium rot on garlic |
FUSARIUM ROT ON GARLIC (Fusarium oxysporum f. sp. cepae)
Fusarium rot superficially looks like bloat nematode damage. The pathogen invades the basal plate and the roots decay and die. If the infection took place late in the season, then you may not see symptoms until the garlic is in storage.
Fusarium is a fungus that persists in soils as resting spores. Warm, moist soil favors
development of the disease. Under very moist conditions a pinkish-white mycelium may develop making the symptoms a bit like white rot. If you are unsure whether your problem is the bloat nematode, white rot, or fusarium rot then you should send a sample into the UMaine Plant Pathogen Diagnostic Lab for identification.
If you have a fusarium problem, then a three year rotation to crops other than any Allium is recommended.
Garlic should be stored in a cool (32-36 degrees F.), dry place in order to slow the development of the disease.
See MOFGA fact sheet #15: Storing Garden Vegetables
 |
| White rot on garlic |
WHITE ROT OF GARLIC (Sclerotium cepivorum)
White rot is a very serious problem because it may spread fast, and once in a field it can persist for many years. Luckily it is a spotty disease and at this time is only present in a small number of fields around the northeast. Those farms can no longer grow any Allium crops in the infested fields.
White rot is one of the most destructive fungal diseases affecting the onion family. It is only a problem on crops in the onion family. (It is not the same pathogen as white mold, which attacks many other crops such as beans, carrots, lettuce, tomato, peppers and more.)
Symptoms of white rot on the leaves include premature yellowing and dying of the older leaves and then death of the plant. That is not much different than many other garlic problems so is not a very useful diagnostic symptom, but the white, fluffy fungal growth (mycelia) on the root end of the bulb is the give away. Eventually this fungal growth moves around the bulb and inward between the storage leaves of onion and cloves of garlic. Small, black sclerotia (tiny hard, black bodies of dormant mycelia) form in the decaying tissue and throughout the white fluffy mycelia. Secondary infections by other fungi may occur.
The pathogen is not known to produce spores. This fungus reproduces only by forming sclerotia, and it also spreads by direct contact, i.e., when the mycelium growing on one plant reaches the roots of the neighbor plant in the row. The sclerotia can lie dormant in the soil for many years until roots of a host plant grows nearby and the sclerotia are stimulated to germinate (see below). Dragging the pathogen around on boots, tillage or other equipment, or moving with soil in heavy rains are other ways the disease spreads. Also, animals feeding on diseased bulbs can defecate viable sclerotia.
The best control is good sanitation. Use clean seed cloves for garlic and clean onion sets and transplants. If only a small number of plants are infected, which is usually the case the first year it is found on a farm, pull the infected plants and destroy before sclerotia are formed.
An interesting idea for speeding up the eradication of white rot sclerotia from the soil is to stimulate them to germinate but not have a crop of Alliums for them. Sclerotia will sit waiting in the soil for 20 or more years until a signal is received that onions or garlic are growing nearby. The growing Allium releases a chemical that the sclerotia can sense. Over the past decade researchers have been studying methods that stimulate the sclerotia to germinate but, of course, with no Allium crop growing for the pathogen to complete its life cycle. This “biostimulation” reduces the number of sclerotia in the soil. There is not a specific recommendation yet, but a few things that may reduce sclerotia in the soil include:
- growing scallions which stimulate the sclerotia to germinate, but the scallions are harvested before the disease completes its life cycle
- making compost out of onion or garlic waste and spreading that in the spring or fall repeated for a bunch of years before trying to grow an Allium again
- making a sprayable concoction from ground up onion or garlic waste, or using garlic powder as a soil amendment for a few years before trying to go back to an Allium
If the disease is known to be present, or if onions from other farms are being stored and packed, equipment, storage bins, etc. should be thoroughly pressure-washed and then disinfested for ten minutes with a 0.5% solution of sodium or calcium hypochlorite, (for example, 1:10 dilution of a household bleach). Then rinse with potable water.
Seed producers should execute extra diligence and may want to regularly disinfect any surface in contact with garlic.
Since chlorine materials will be inactivated by organic matter stuck on boots, quaternary ammonium compounds may be used as boot dips inside storage areas and packing sheds, and before and after leaving fields. Quaternary should not be used on any apparatus that is in direct contact with the garlic or onions or any other crop. Disposal of the dip solution must be in a manner that does not contaminate the soil, water or crop. Note: not all quaternary ammonium products are labeled for boot washes so read the label.
At this time, no organic material has shown efficacy in controlling this fungus.
 |
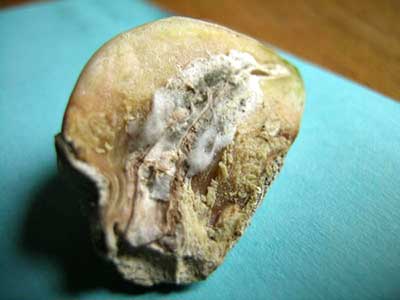
| Botrytis porri on garlic |
BOTRYTIS ROT
Botrytis rot of garlic is caused by the species, Botrytis porri, which is a different species of the very common genus that includes the species Botrytis cinerea, which causes gray mold. It is not common but sometimes does incite severe disease and may cause major crop loss.
In the field, garlic plants infected with Botrytis rot are stunted and have dead or dying outer leaves. Since most garlic diseases have similar symptoms, it is important to send a sample to the UMaine Plant Disease Diagnostic Lab for a positive ID.
Infection develops at soil level and initially looks water soaked but later becomes dry and brown. If conditions are not favorable for the fungus, plants infected with Botrytis porri sometimes recover. If conditions are favorable for fungal growth, and in plants infected later in the season, distinctively large (up to 2 cm) black sclerotia form around the rotting neck of the garlic. At this point the disease is clearly identifiable. Sclerotia are hardened bits of fungal mycelia that are very resistant to environmental stresses of wet, cold, dry and hot. In other words, sclerotia are tough and can survive almost anything in nature. The sclerotia overwinter, and in spring they produce spores that start the cycle again.
Under cool, wet conditions, the disease can become epidemic in a field. It does not progress when the weather is warm and dry; in such conditions, proper irrigation is important in preventing the disease from spreading, so don’t keep fields excessively wet. Good air flow is important, so avoid weedy fields and don’t crowd plants. Excessive nitrogen may also be implicated in susceptibility. Clean seed and crop rotation also help prevent Botrytis rot.
 |
| Penicillium decay on garlic |
PENICILLIUM DECAY
Penicillium decay may be responsible for poor plant stands in the field but is better known for causing garlic to decay in storage. It is one of the most common storage problems and arises when the harvested garlic does not dry quickly and well. The common name, blue mold, describes the most obvious symptom: The fungus frequently produces a mass of blue spores on the surface of decaying cloves. Spores are not always produced; sometimes, especially early in its growth and under drier conditions, you will see only white mycelia (a mass of fungal filaments) growing on the surface of cloves. Frequently, pencillium rot affects only a few cloves in a head. I have gotten suspicious heads of garlic to sporulate by placing them in a plastic bag for a few days; when the blue spores form, I know the disease is present. Symptoms in the field include wilted or stunted seedlings due to decay. The fungus can spread into the stem plate of young plants, affect the development of roots and result in weak, yellow leaves.
The pathogen is usually Penicillium hirsutum. Inoculum survives from year to year in infected cloves. It does not survive well in soil, so clean seed and crop rotation together very effectively minimize the problem. Even within a single head, some cloves may be infected while others are clean – but when you break heads apart to plant the seed, Penicillium spores are inevitably spread to other cloves, and infection occurs through small wounds. To minimize spreading the problem when you see a few infected cloves, handle the seed carefully to minimize wounding, and plant the seed as soon as possible after separating the cloves from the bulb.

This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, through the Northeast Sustainable Agriculture Research and Education program under subaward number ONE19-334.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
