by Eric Sideman, PhD
MOFGA’s Organic Crop Specialist Emeritus
Nitrogen is usually the nutrient that is in limiting supply, i.e., the limiting factor to crop growth on organic farms. Plants deficient in nitrogen are stunted, yellowish (especially the lower leaves), and have restricted root growth. Plants turn yellow because nitrogen is an integral part of chlorophyll, which is the chemical that makes plants green. In plant cells chlorophyll captures light energy and changes it to chemical energy. Nitrogen is also part of nucleic acids, enzymes, and all other proteins so it may limit growth and production without showing deficiency symptoms. Of all the soil minerals, nitrogen fertilization usually has the quickest and most pronounced increase in plant growth and yields.
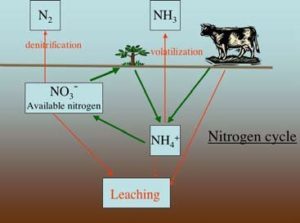
One of the reasons nitrogen is found only at low levels in soil is that soil parent materials usually contain little or no nitrogen. Nitrogen enters the soil system by biological activity. Certain species of bacteria are able to take nitrogen from the vast supply in the atmosphere (approximately 80%). Some of these bacteria are free living in the soil and some form symbiotic relations with particular species of plants, many of which are in the legume family. Plants, on their own, are unable to tap the atmosphere for nitrogen. Rather they are dependent on nitrogen ions released from the decay of the organic chemicals originally produced by nitrogen fixing bacteria.
Figure 1 illustrates the cycling of nitrogen through the farm and garden ecosystem. The inner set of arrows illustrate the desired cycle beginning with nitrogen fixation by the bacteria and cycling around through decomposition of organic matter. In addition to fixation taking place in your fields nitrogen can be added to the system from previously fixed sources such as crop residue, manure, and fertilizer. The outer arrows illustrate avenues of nitrogen loss from the system. Nitrogen is lost from soil through leaching, denitrification and volatilization, which are all explained below in detail.
Nitrogen additions to farm and garden soil are crucial for sustained production. On the other hand, oversupplying nitrogen results in environmental problems. Oversupplying nitrogen is rarely a problem on organic farms except when there is a cheap supply of materials such as manure. Many forms of nitrogen are soluble and while immediately available to plants they are also subject to washing away from the farm or garden and end up in wells as dangerous nitrates or as pollution in lakes and coastal bays. An oversupply may delay crop maturation by encouraging excessive vegetative growth, may adversely affect fruit and grain quality, and it may reduce resistance to plant diseases or attract insects such as aphids. Nitrogen in stable forms such as compost raises less risk of these detrimental affects. Nitrogen in very soluble forms, such as synthetic fertilizers or chicken manure, raises the greatest risk of pollution and crop and soil damage.
ECOLOGICAL NITROGEN MANAGEMENT
The ecological impact of a farm or garden is often measured by the amount of nutrients crossing the farm or garden boundary, both in and out. Systems with the lowest impact emphasize the conservation and cycling of nutrients within fields and between fields of the farm or garden system, and only use nutrients from outside the system to supplement crop needs. Systems dependent on industrially fixed nitrogen, as well as those that make up for nutrients lost from the system by bringing in more, have the highest impact. Organic farming and gardening should be based on a system of low environmental impact. The National Organic Program Rules require this. However, I frequently see some folks who call themselves organic who forget this and simply substitute natural sources of nutrients for synthetic ones even though the environmental costs of misusing natural fertilizer may be just as high as being dependent on ammonium nitrate or urea.
Optimal nitrogen management is based on biological nitrogen fixation and minimizing nitrogen losses from the system. The system with the least risk of environmental impact is one that gets its nitrogen from crop rotations with legume green manures. The green manures incorporate the fixed nitrogen in their tissue and release it when they decompose to either be picked up by the next crop or held in the stable organic matter of the soil (humus). Farms typically use one to five years of legume cover crop growth to fix enough nitrogen for one to three years of cash crop growth. The rotation chosen is not only based on nitrogen needs but on many other factors such as building soil organic matter and biological activity, weed control, and managing other nutrients.
In most systems nitrogen from rotation with legumes is supplemented with nitrogen from other sources. But I stress that the farms and gardens that use rotations with nitrogen fixers have less environmental impact than farms buying in soil amendments for the bulk of their nitrogen needs. So the goal is to minimize the amount of nitrogen needed from outside the farm.
Supplemental nitrogen sources in organic systems include manures, compost, and other plant and animal products such as seed meals, blood meal, or legume hay meals. Before I compare these I want to discuss the potential losses of nitrogen in more detail.
Potential Loss of Nitrogen
The nitrogen in animal and vegetable residue for the most part is combined in complex proteineous compounds in the plant or animal tissue. Decomposition by feeding bacteria converts the nitrogen to a soluble form that can be utilized by plants. As seen in Figure 1 this is a two-step process. The first step is mineralization where the feeding bacteria release ammonium ions (NH4 +). In the second step, called nitrification, the ammonium ions are used by other bacteria, which release nitrate ions (NO3 – ). The nitrate ion is the form most plants utilize. Some plants, such as blueberries, can easily use the ammonium ion.
In an ideal system this cycle would be all of the story, but the real system is not ideal. Nitrogen can be, and to a greater or lesser extent is, lost from the system at a few points. Under conditions where the nitrification bacteria are not working hard such as high pH, low soil temperatures, and low oxygen levels (such as waterlogged soils), the ammonium ions turn into ammonia gas and drift off into the air (volatilization). Most of you have smelled this in a barn or from a pile of fresh grass clippings. If nitrification takes place and then the system becomes anaerobic, denitrification occurs. Denitrification is the conversion of nitrate ions to nitrogen gas before plants can pick them up. The other major loss of nitrogen from the soil is by leaching. The nitrate ion is very soluble and is not held by the soil so if no plants are growing rapidly at the time when the nitrate ions are available, they will be washed down into the ground water.
All of these possibilities must be considered when adding nitrogen to a cropping system. If crops are growing rapidly then you should plan to have nitrate ions becoming available from decomposing crop residue or added in a form that will become available quickly. If no crops are growing there should not be an abundance of nitrate ions added because they will be lost, wasted and end up polluting surrounding environments such as ground water. These guidelines apply no matter what the source of nitrogen is. For example, nitrogen rich legume cover crops should not be plowed down without soon afterwards planting a nitrogen demanding crop. Similarly, if nitrate ions remain plentiful at the end of the growing season then a nitrogen demanding cover crop should be planted to catch these before they leach away.
SUPPLEMENTING NITROGEN WITH ORGANIC FERTILIZERS
If nitrogen is needed quickly to support a rapidly growing crop, then a source must be chosen that decomposes quickly to make nitrate ions available. On the other hand, if you are adding fertilizer in the spring and planting crops that will not have a high nitrogen demand until mid summer, a slower decomposing organic fertilizer may be chosen. The table below gives you some summarized information for supplementing your nitrogen with a natural fertilizer.
The information summarized here can help you decide which is the best nitrogen source for a particular situation. First, total nitrogen ranges from 1.45-13.8% for the materials listed. Even the 2.8% in alfalfa meal is relatively high for organic nitrogen sources. For example, most compost is about 1% nitrogen.
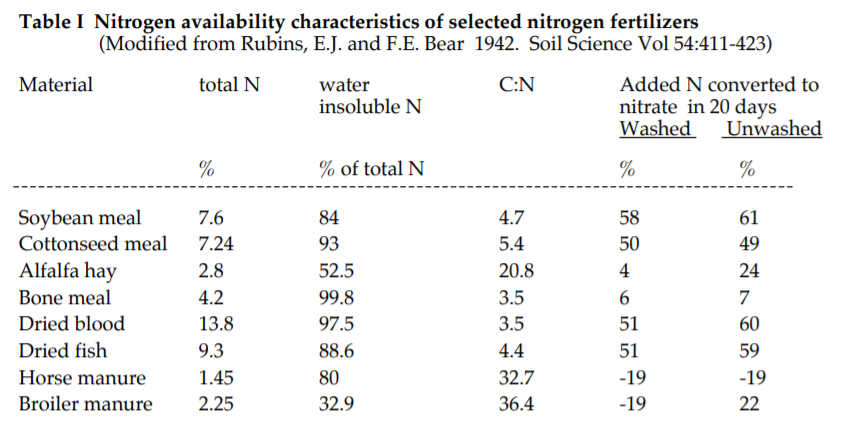
The availability of the nitrogen is just as important as the total nitrogen when deciding which material to use. Availability is based on a number of characteristics of the material as well as the soil and weather. If most of the nitrogen in a material is not water soluble that means it is likely tied up in proteins or other complex compounds and will depend on biological activity to be made available. Note in the table that this is so for all the materials except for chicken manure. Chicken manure has a great deal of water soluble nitrogen compounds (only 32.9% is insoluble) and thus is similar to synthetic nitrogen fertilizer in that a lot of the nitrogen is immediately available to the plants. But, this also means that the nitrogen in chicken manure is very likely to be lost to leaching or volatilization if mishandled. This explains why the percent of the total nitrogen converted to nitrate in 20 days is negative for the washed chicken manure…the nitrogen was washed away.
Most natural sources of nitrogen depend on the activity of decomposer bacteria to make the nitrogen available to plants as nitrate ions. The carbon to nitrogen ratio (C:N) is the major determining factor. The “C” in this ratio refers to energy rich carbon containing compounds that are low in protein, such as carbohydrates. The “N” refers to nitrogen rich compounds such as proteins. As a general rule, the larger the C:N ratio of a substance, the less immediately available to plants is the nitrogen. This is because the bacteria that are feeding on the material use up all what available nitrogen there is for their own needs while feeding on all that rich carbonaceous material. In addition, the easier the carbonaceous material is to decompose, the less nitrate accumulates, e.g., if a material has a high C:N ratio and is made of easily decomposed carbonaceous compounds the nitrate will accumulate very slowly because the bacteria will assimilate it as quickly as it is created. Horse manure, because it typically has lots of sawdust as bedding, is an example of a material with a high C:N ratio. The decomposer bacteria actually grab nitrogen from the surroundings, as well as all the nitrogen in the manure, in order to meet the needs of their very fast growth. That is why there is a negative 19 for horse manure in the nitrate column of the above table. Dry blood is at the other extreme. It has a very low C:N ratio, a very high total nitrogen percentage, and decomposes very quickly in soil. As you can see in the table, more than half of its 13.8% of nitrogen will be converted to nitrate in 20 days.
The low availability of nitrate from bone meal is due to a different mechanism. As you can see, even though it has a relatively narrow C:N ratio (because there is very little carbonaceous material in bone) the nitrogen does not quickly become available nitrate. The nitrogen compounds in bone meal are simply very resistant to microbial decomposition.
WHICH MATERIAL SHOULD YOU USE WHEN?
All of the materials listed in the table, except for the manures, are usually considered too expensive to broadcast over a whole field before planting. Furthermore, adding a lot of nitrogen fertilizer over a field fertilizes more weeds than crop. These materials are better incorporated into the rows or beds where the crops are actually growing. Some of these release the nitrogen in an available form so quickly they should only be used when a crop is ready to pick it up. For example, side dressing foot-tall corn in a garden with dried blood is a good way for an organic grower to quickly make up for a deficiency. But the nitrate is released so quickly that much of it is subject to leaching, similar to using synthetic fertilizer. It is also a very expensive source of nitrogen.

Manures, fish meal and the seed meals (cottonseed and soybean) are good sources of nitrogen to use with crops needing more nitrogen than you can supply with your rotation. However, you must have a crop ready soon to capture the nitrate as they have relatively low C:N ratios and produce nitrate more quickly than something like alfalfa pellets. Alfalfa pellets have a moderate C:N ratio and release nitrate at a steady rate over the season. Economically, it is a good source, even though it is on the low end of the materials presented in the table.
Determining how much nitrogen to add in an organic system is very difficult. The Maine Soil Testing Lab does not measure available nitrogen for their standard soil test because it is impossible to take into account all of the variable factors such as weather. The available nitrogen from organic sources fluctuates daily with weather and consequent biological activity. A conservative application rate based on what typical crops remove and what nitrogen is available from existing sources, such as soil organic matter, is 20-50 lb/A of nitrogen (.5- 1.25 lbs/1000 sq ft). Soluble fertilizer recommendations are typically more than double this because so much is lost. Your best bet is to keep good records of your crop growth in your own situation and come up with your own needs.Recently, farmers are testing soil to determine if their system is providing enough nitrogen using something called the “Pre side dress Soil Nitrate Test” (PSNT). The PSNT is a soil nitrate test that was originally developed to help make an accurate nitrogen fertilizer recommendation for a corn crop that has had an application of manure, a cover crop that contributes nitrogen to the soil, or has had a previous application of nitrogen from another source. Research has been done in several states that shows when 25 ppm nitrate-N is present in the top foot of soil when the corn is about a foot tall, response to additional side dress N is not likely. This has saved a lot of farmers from wasting lots of fertilizer and impacting the environment. Although recent work has shown that the test also works for many types of crops, it is not perfected yet for all crops. Contact your local Extension office for up to date recommendations on using the PSNT for your crops.
Last published November 2007
