by Eric Sideman, PhD
MOFGA’s Organic Crop Specialist Emeritus
and David Colson, New Leaf Farm
Introduction
The soil is the source of all but a very small part of a plant’s nutrition. Soil testing is a quick way to assess the nutrient status of your soil and to determine what amendments are needed for an optimal yield of your crop. This fact sheet should help you understand the soil test results and determine which soil amendments to apply.
There are two basic approaches to fertilization. The first is to provide required nutrients to each crop in a soluble form that plants can use immediately. In other words, feed the plants directly. The advantage of this approach is the opportunity to quite accurately meet a crop’s needs. The disadvantage is soluble nutrients do little to build long term soil fertility and often leach and run off soils to end up as pollution.
The second approach to fertilization is building and maintaining stable nutrient levels in the soil that in turn can provide a crop with its needs. Sustainable soil fertility is based upon conserving nutrient, maintaining organic matter and promoting soil microbiological activity. This is accomplished by practices including adding slow release, finely ground, mineral rich rock powders and compost and using cover crops in rotation with your cash crops. The advantage of this approach is continued improvement of soil fertility, protection of natural physical and biological processes in the soil, and minimal environmental disturbance. Building long-term soil fertility is the kingpin of organic farming and gardening. However, it is not as exact a science as providing soluble nutrients and it is difficult to determine precise amounts of organic soil amendments.
Certain elements – calcium, magnesium, potassium, nitrogen, phosphorus, and sulfur that are required by plants in relatively large amounts are called macronutrients. Others are required in very small quantities. These micronutrients will usually be adequately supplied if the pH of the soil is in the appropriate range for the particular crop and there is an adequate amount of organic matter in the soil. The need for supplemental application of micronutrients is best gauged after optimal levels of macronutrients have been developed. A standard soil test only addresses macronutrients. Three years of soil testing will give the grower a good picture of the success of a soil-building program.
Contact your local Extension Office for a soil testing kit.
Interpreting the Results
What’s there. (See the sample soil test report on the following page). The laboratory results at the bottom of the form give the following:
- Soil pH
- Available Phosphorus (P), Potassium (K), Magnesium (Mg), and Calcium (Ca), as pounds per acre
- Cation exchange capacity (CEC) at the projected pH management level
- The percent saturation of the soil CEC with K, Mg, Ca, and acidity
The bar graph above the numerical lab results enables you to determine fertility status of the soil at a glance.Beneath the bar graph are recommendations for addition of soil amendments. The recommendations are theoretically calculated nutrient requirements needed to supplement the present available level in the soil in order to feed the particular crop and reach a target yield. The recommendations are based on the first of the two approaches to fertility discussed above, in other words, feeding the crop.
Many organic farmers ignore the recommendations and use the soil test form only as a measure of their soil building efforts. Others will concentrate on their soil building but will also attempt to meet yearly-recommended crop needs with organic soil amendments. This will probably give the greatest yield, but is often very expensive.

Understanding the Results
- pH
This is a symbol denoting the relative concentration of hydrogen ions (H) in the soil solution. Soil pH values in Maine range from 4.5 to 8.0. A pH of 7 is considered neutral. pH values below 7 are called acid and those above 7 are called alkaline. Vegetables generally do well in soils 6.0 (slightly acid) to 7.2 (slightly alkaline). Some plants, such as blueberries do well in very acid soils.
The major reason pH is important is because different nutrients become less available to plants in acid or alkaline soils. The first step when improving soil fertility should be correcting the pH.
- Phosphorus (P)
This figure is a measurement of the amount of phosphorus in the soil, which should be available for plant uptake over the course of the next growing season. Many soils have a great deal of additional phosphorus tied up in complex mineral compounds that hold it unavailable to plants. Over time, chemical and biological activities in the soil free a portion of this phosphorus. Free phosphorus is quickly bound up by soil. It also has very low mobility. Any phosphorus picked up by plants usually comes from soil very near the roots. Placement of phosphorus fertilizers in the root zone is important.
Since most soils have about the same ability to hold phosphorus (unlike the cation nutrients discussed below) the actual pound per acre of available phosphorus is an important number.
Although it varies from crop to crop, general guidelines are:
< 5 Low
05 – 10 Medium
10 – 20 Satisfactory
20 – 35 Optimum
> 40 Excessive
- Potassium (K), Magnesium (Mg), Calcium (Ca), and CEC
Measurements of these nutrients are first given as pounds per acre available for plant uptake. However, these numbers are not as important to the actual availability as is the case with phosphorus. The complicating factor has to do with the varying capacity of soils to hold and release these nutrients, which is called the CEC (cation exchange capacity).
Potassium, magnesium, calcium, and hydrogen are cations (positively charged ions). These are held in the soil at negatively charged sites. The more negatively charged sites (high CEC) in the soil, the more cations the soil is able to hold. A soil with a high CEC (15) has the potential of being very fertile, i.e., having a great reservoir of nutrient cations. A soil with a low (CEC) (<7) cannot hold a large reservoir.
The potential for high fertility measured as CEC is based upon soil texture, pH and organic matter content. Clay soils and soils with high organic matter content tend to have high CEC and potentially can hold high levels of cation nutrients. Sandy soils have low CEC and tend to lose cation nutrients to leaching. CEC also increases with pH.
A Soil with a high CEC may still have low cation nutrient fertility, even if the pounds per acre are relatively high. Such a situation is possible if the CEC sites are filled with non-nutrient cations such as aluminum or hydrogen. Conversely, a soil with a medium CEC and only moderate pounds per acre of nutrient cations may be fine if the sites are filled with nutrient cations in the proper relative proportions.
Percent Saturation (printed after CEC on the result form) is a better means of evaluating nutrient balance of cation nutrients in soil than pounds per acre because it shows the relative level at which various nutrients occupy the soil CEC, and consequently their availability to plants.
Desirable ranges of % saturation:
K 3.5 – 5%
Mg 10 – 25% (should be twice K)
Ca 60 – 80%
Acidity (represents non-nutrient cations, Al and H) < 10%
The nutrient balance is very important because very high concentration of one cation in the soil can adversely effect the uptake of other cations. For example, excessive calcium (Ca) can induce a magnesium (Mg) deficiency and can also reduce phosphorus availability. Excessive potassium (K) can also suppress magnesium (Mg) uptake.
Sources of Nutrients to Meet Recommendations
- pH
Most soils in Maine are naturally acidic and limit plant growth. The most common way to adjust pH is by the addition of limestone as directed in the recommended section of the soil test results.
Wood ash can also be used. It has about 2/3 the liming value of limestone, and also is a source of potassium.
- Phosphorus
There are at least five common sources of phosphorus:
- Native compounds in soil, both organic and inorganic
- Rock Powder
- Barnyard manure
- Plant residues including green manure, and
- Commercial fertilizers including bone meal
Increasing the availability of native soil phosphorus is the first step in building phosphorus fertility. The availability of phosphorus is largely determined by soil pH. Both acid and alkaline soil severely limit the availability of phosphorus. Adjusting pH should be the first step before paying for phosphorus amendments. Phosphorus is most available at pH 6.5 – 6.8.
Organic matter is a major source of phosphorus for organic farmers. Phosphorus released by decaying residues is highly available, but its release is dependent on high levels of biological activity. Consequently, maintaining high organic matter levels and feeding the biological activity by green manuring may be more appropriate for your system in building phosphorus fertility than paying for soil amendments. (see MOFGA Bulletin and Fact Sheet on green manures).
Colloidal rock phosphate (2% available, 18% total phosphorus) is a common source of phosphorus on organic farms because it is the least expensive natural source. The drawback is that only about 2% of the phosphorus is available. After the pH has been adjusted and it is determined that phosphorus is needed the initial application of colloidal rock phosphate should be:
- If soil Phosphorus is < 5 lbs. /acre, add 2 tons/acre (90 lbs. / 1,000 square feet)
- If soil Phosphorus is 10 – 20 lbs. /acre, add 1/2 – 1 ton /acre (20 – 40 lbs. / 1,000 square feet)
Once the soil test shows available P is 20 lbs. per acre or more, adding 1/2 ton per acre every four years should maintain an optimum level. However, maintaining phosphorus levels through organic matter addition is possible and may be more desirable.
Bone Meal (11% available) is the most common source of immediately available phosphorus for organic farmers. Generally rock or colloidal phosphate is used for long-term improvement and bone meal is used to meet immediate demands. But anything more than side dressing would be too expensive.
Poultry manure (1% available in fresh manure) is messy and can pollute because of so much soluble nitrogen but it is a very good source of quickly available phosphorus. Initial application to soils low in phosphorus is 5 tons per acre.
Potassium
The most common source of potassium on organic farms is SulPo-Mag, which is not a slow release source but a natural rock powder with soluble potassium (21%) and Magnesium (11%). You can use Sul-Po-Mag to meet K recommendations as follows, if you also need Mg.
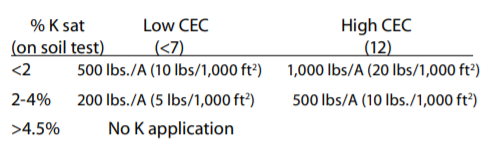
In practice, once a good balance of potassium and other cations is reached the liberal use of plant residues and manure (except poultry) supplies enough potassium.
Granite dust and greensand are rock powders that have significant levels of potassium that is released slowly. Initial applications should be 2-3 tons per acre depending on level of fertility.
Wood ashes are also a good source of potassium (5%). They should be stored dry through the winter and spread in the spring to avoid loss of K by leaching. Do not use wood ash unless you need to adjust the pH in addition to adding potassium.
Magnesium and Calcium
Magnesium and calcium are usually applied together as dolomitic limestone. If no magnesium is wanted, use calcitic limestone to adjust pH. If magnesium is needed without pH change use Sul-Po-Mag for potassium and magnesium. If magnesium is needed and pH is good and no potassium is needed then Epsom salts may be used.
Nitrogen
No test results of nitrogen are included because three is no good way to measure soil nitrogen. Nitrogen availability varies widely from week to week as a result of biological activity and weather conditions.
The recommendations in the soil test results are based on the crop needs and not on the soil’s nitrogen supplying capacity.
An organic grower must take into account the nitrogen supplied from addition of plant residues, barnyard manures, green manures, and residual soil organic matter. Rotating with legumes and adding of manure and compost is the desired methods of supplying nitrogen. Observation of plant growth is the best indicator of N needs. The following tables are a rough guide to nitrogen fertilization. Account for phosphorus and potassium content of these amendments when figuring P and K needs. For a detailed discussion of nitrogen management in agricultural soils see MOFGA Fact Sheet # 8, Providing Nitrogen to Organic Crops.
Table I. Suggested nitrogen fertilizer rates for average yields of some common crops in Maine (assuming residue is left in field)
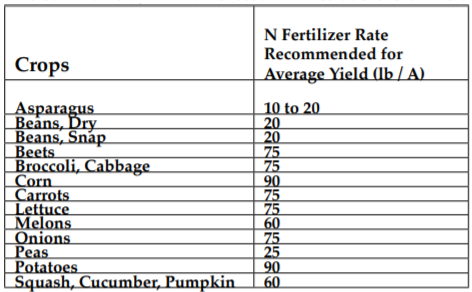
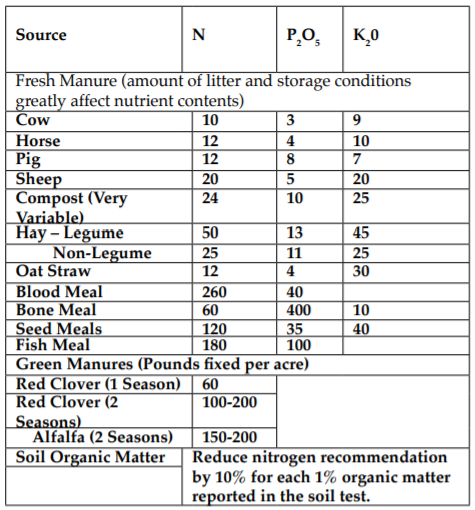
Table II. Estimate of available nitrogen, phosphorus, and potassium from common organic sources (lbs / T)
Want to know more?
For more detailed infmation about soil fertility see:
Fertile Soil by Robert Parnes
Building Soils for Better Crops by Fred Magdoff and Harold van Es
