 |
| Nitrogen deficiency often appears as a uniform yellowing on the lower leaves of plants. English photo. |
By Eric Sideman, Ph.D.
Nitrogen (N) is the nutrient most commonly limiting crop growth and yield on organic farms. This is especially true when creating a farm from an old, abandoned field and when transitioning from conventional to organic fertilizing practices, because N, unless managed, is easily lost from soil. Also, unlike other nutrients needed for plant growth, the parent minerals that make up soil contain little or no N; N found in soils comes from biological activity.
Low levels of N will lead to smaller crops and lower yields. Severely N-deficient plants are stunted, yellowish (especially the lower leaves) and have restricted root growth. Plants turn yellow because N is part of chlorophyll, the chemical that makes plants green. Lower leaves turn yellow first because N is a “mobile” nutrient – one that plants can easily move around; so when levels are low, the plant moves N from older leaves to the demanding, faster-growing tips.
The Nitrogen Cycle
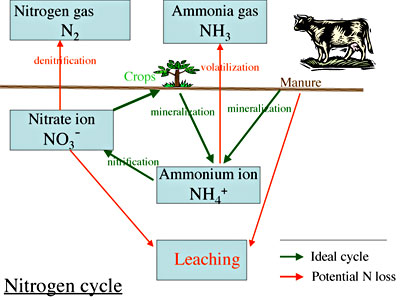
The first step to learning how to manage N on a farm, especially an organic farm where you cannot simply buy a bag of synthetic N, is to understand how N cycles. Figure 1 illustrates how N cycles through the farm and garden ecosystem. The green arrows illustrate the desired cycle.
Nitrogen is integral to all proteins, so it is found to a greater or lesser degree in organic matter. Organic matter (OM) containing lots of protein (e.g., fish waste, manure) is high in N, while organic matter with little protein (e.g. wood chips) is low in N. Organic farmers add N to their cropping systems by adding different forms of OM (compost, manure, seed meals, etc.) or growing legume cover crops (see Box I).
 |
| Figure 1 |
The green arrows in Figure 1 show how manure or crop debris decomposes into a form of N that plants can absorb. Proteins, which are not water soluble, are not available to plants. Soil bacteria metabolize proteins and leave behind as their waste chemicals that plants can take up.
The first set of bacteria to metabolize proteins leaves an ammonium ion behind. Then a second set of bacteria uses this ammonium ion for food and leaves nitrate ions behind as its waste. Most plant species can take up these nitrate ions from the soil. A few species, such as blueberries, can easily use ammonium ions. Not surprisingly, conventional growers generally buy chemicals that are simply nitrate salts, such as calcium nitrate, potassium nitrate or ammonium nitrate.
Potential Losses of Nitrogen
The red arrows in Figure 1 illustrate different ways N is lost from the system, depending on its chemical form. Most N in animal and vegetable residue is combined in complex protein compounds. As noted, when bacteria decompose protein, they convert the N tied up in proteins to a soluble, active form. Figure 1 shows this two-step process. In the first step, mineralization, the feeding bacteria release ammonium ions (NH4+). In the second, nitrification, other bacteria use ammonium ions and release nitrate ions (NO3-).
| Nitrogen Fixation by Legumes
The atmosphere is 80 percent N gas, but this N is present in the atmosphere as a stable molecule of two N atoms (N2) tightly bonded together. Plants and animals cannot use N2 to make amino acids (and then proteins) because they cannot split the two N atoms apart. Instead, plants get their N from ions – primarily nitrate – in the soil solution. Plants and animals depend on a few species of bacteria that can “fix” N gas; i.e., that can cleave the N2 molecule in the air to build biologically useful molecules. Some of these N fixing bacteria are free living in the soil; others live symbiotically with plants, especially plants in the legume family. Farmers grow legumes as cover crops because the N fixed from atmospheric N2 gas is then added to the soil and becomes available to future crops when the legume is plowed in and decomposes. |
In an ideal system this cycle would be the whole story, but the real system is not ideal. When N is part of proteins, it is quite resistant to being lost, but when bacteria decompose protein, the N is susceptible to loss. Preventing protein decomposition is one way to conserve N and save it for later use. Drying seed meals and holding manures in anaerobic conditions such as manure pits or hard packs are examples of preventing decomposition. But once protein molecules are decomposed, N can convert to gases lost to the air or to soluble forms lost in moving water. These losses waste N and can create environmental problems.
Under some conditions, such as high pH, low soil temperatures, and low oxygen levels (e.g., waterlogged soils), proteins may be decomposed and the ammonium ion may form, but because the nitrification bacteria are not converting ammonium to nitrate, the ammonium ions become ammonia gas, which goes into the air (volatilization). You may have smelled this in a barn with wet bedding or coming from a pile of fresh grass clippings.
If nitrification occurs and then the system becomes anaerobic, denitrification occurs – i.e., nitrate ions are converted to N2 gas before plants can absorb them, so most of your valuable fertility is lost to the air. We all have experienced this loss of N in recent years when, after a nice spring of soil prep, June brings torrential, endless rain.
The other major loss of soil N is by leaching. The negatively charged nitrate ion is very soluble and is not held by cation exchange sites in the soil. If no plants are growing rapidly when the nitrate ions are available, and there is lots of rain, nitrates will be washed, or leached, down through the soil.
Consider all of these possibilities when adding N to a cropping system. When crops are growing rapidly, plan to have nitrate ions becoming available from decomposing crop residue or added in a form that will become available quickly. If no crops are growing, do not add an abundance of nitrate ions, because they will be lost, wasted and pollute surrounding environments such as ground water. These guidelines apply regardless of the N source. For example, N-rich legume cover crops should be plowed down only when an N-demanding crop will be planted soon. Similarly, if nitrate ions remain plentiful at the end of the growing season, plant an N-demanding cover crop to catch these before they leach. The cover crop can hold them over winter; when it decomposes in spring, it will release N for spring cash crops.
How Much Nitrogen to Add?
Organic fertility management is much less exact than feeding plants with synthetic chemical fertilizers. Organic farming practices center on conserving and recycling nutrients through soil biological activity. Fertilizer recommendations cannot be exact because so many factors affecting nutrient cycling, e.g., temperature, moisture, variable nutrient content of the input, are not accurately predictable. Although you can determine how much N is in a manure, compost, seed meal, etc., predicting how much will be mineralized and converted to nitrate for plant uptake, and how much will be lost, is not an exact science. In addition to environmental factors and total N in the material, the chemical makeup of the material is important too. The N in some materials is part of complex molecules that take longer to decompose than simple molecules in other materials. Table I shows how much of the total N in some common organic fertilizers converts to nitrate in 20 days.
Table I. Nitrogen availability characteristics of selected nitrogen fertilizers
(Modified from Rubins, E.J. and F.E. Bear, 1942. Soil Science, Vol. 54:411-423)
| Material | Total N | Water insoluble N |
C:N | Added N converted to nitrate in 20 days |
|
| Washed | Unwashed | ||||
| % | % of total N | % | % | ||
| Soybean meal | 7.6 | 84 | 4.7 | 58 | 61 |
| Cottonseed meal | 7.24 | 93 | 5.4 | 50 | 49 |
| Alfalfa hay | 2.8 | 52.5 | 20.8 | 4 | 24 |
| Bone meal | 4.2 | 99.8 | 3.5 | 6 | 7 |
| Dried blood | 13.8 | 97.5 | 3.5 | 51 | 60 |
| Dried fish | 9.3 | 88.6 | 4.4 | 51 | 59 |
| Horse manure | 1.45 | 80 | 32.7 | -19 | -19 |
| Broiler manure | 2.25 | 32.9 | 36.4 | -19 | 22 |
Remember, although this table makes N availability look like an exact science, it really demonstrates another factor of the complexity of predicting how much fertility the plant is actually getting from soil amendments. The key to good nutrient management on organic farms is to have reservoirs of nutrients large enough to support good crop growth but not so large that they pollute soil or water. Knowing how biological cycles work and how potential N losses occur helps you figure N amendment additions, but you’d have a false sense of security if you thought you could meet crop demands exactly with precise amounts of organic soil amendments. All of this supports Robert Rodale’s old slogan: Feed the soil, not the crop. Organic farming is all about building better soil.
Estimating Nitrogen Additions
From soil organic matter
When calculating how much N to add, consider how much is already in your soil. Organic matter left in the soil from previous applications and crop debris decomposes and releases nitrogen. Estimate the total N per acre in the soil’s root zone by multiplying the soil OM content by 1,000. Soil OM is reported on a standard UMaine soil test report. For example, a soil with 4 percent OM contains about 4,000 pounds of N per acre. About 1 to 4 percent of this total N is decomposed into available forms per year. Thus, in a soil with 4 percent OM, about 40 to 160 pounds of N should become available to crops, depending on whether conditions such as temperature, moisture, pH, etc., favor bacterial activity. Availability also depends on the type of organic matter. For example, residue from previous years of cow manure applications will release more N than residue from wood chips from horse manure and bedding applications, even though both will show up as OM on a soil test. The important point is that vegetable crops need about 100 to 200 pounds of N per year, so residual soil OM can supply a significant portion of this.
From manures
Manures vary not only in the total amount of N they contain but also in how quickly that N is released, what other benefits the particular manure offers to soil husbandry, and the effects of bedding materials in manures. Poultry manure releases N fastest and is very useful for crops that need a quick boost, but poultry manure is very low in OM, so does little to build soil. Cow and horse manures have significantly less available N but are loaded with OM and are great long-term soil amendments. However, horse manure can have so much bedding that it locks up N, temporarily reducing N availability while bacteria feed on the carbonaceous material in the bedding. These bacteria out-compete the crop for N both in the manure and in the soil, so crops will be deficient. Once bacteria consume the bedding, they die and release N to the soil, but by then the crop has suffered, perhaps for the whole growing season. Also, horses’ inefficient digestion leaves lots of viable weed seeds. Table II shows the average total pounds of N per ton of manure for different livestock, but does not account for the effects of bedding.
Table II. Average pounds of N per ton of manure
| Cow | 11 |
| Horse | 12 |
| Pig | 13 |
| Sheep | 20 |
| Chicken | 30 |
Manure Math
About 50 percent of the total N in fresh cow manure is available to crops during the year of application. That’s about 5 pounds of N per ton applied, or about 100 pounds of N from a 20-ton application.
The year after fresh manure application, N is still being released. Five to 10 percent of the total original N may become available the year after application. So the year after a 20-ton per acre application of fresh cow manure, another 15 pounds or so of N will be available per acre; and two years after application, more will be released, and so forth, with the amount decreasing until the old manure remaining is releasing N at the same low rate as soil OM.
The point is to take all the N into account. In fields where manure has been applied annually over many years, a lot of N will be available, so little or no fertilizer N may be needed. Adding more manure may even pollute waters around the farm.
Poultry manure can supply roughly 3 to 6 times more N per ton than fresh cow manure, or about 15 to 30 pounds of available N per ton per acre in the year of application, depending on its moisture content. With poultry manure, a higher percentage of the total N is converted to plant-available forms in the year of application, leaving relatively less carry-over N for crops in succeeding years.
No matter which manures you use, handle them well to avoid situations that would lead to the N losses discussed above. Don’t let them dry on the soil surface; don’t apply them when no crop will be actively growing; don’t apply them before a predicted heavy rain; don’t apply them to frozen ground, etc. Also, remember the waiting period required in organic standards between manure application and harvesting a crop (90 days, or 120 days if the edible portion of the crop contacts soil).
From green manures
Green manures are cover crops that are turned under to improve soil fertility. (See MOFGA Fact Sheet #10 at www.mofga.org for a detailed discussion of green manures.) Legume cover crops such as alfalfa, cowpeas and hairy vetch can fix 150 pounds of N or more per acre under good conditions. Clovers and field peas generally fix about half that. Remember, N from cover crops is subject to the same rules discussed above relating to the N cycle, so handle them well to avoid N losses. To provide the most N to a subsequent crop, incorporate legumes just before or when they flower.
Several factors can reduce N contributions from legume green manures. If the seed is not properly inoculated, a legume may not form the root nodules necessary for associated bacteria to fix atmospheric N. Clovers, vetches, alfalfa, etc., require different inoculant species. Also, a fair stand of a green manure will provide significantly less N than a good stand. As legumes age and get woodier, the foliage will contain a lower percentage of available N. And if conditions are wet, cool or dry, microbial activity will be reduced and so will the amount of N released via decomposition, and the potential for loss will be increased.
Legume green manures are a great way to obtain N. They fit into a good crop rotation plan, help maintain soil structure and help manage insects and diseases. By growing your own N, you can minimize the need to purchase more expensive N sources. And legume green manures allow you to add N to the farm without adding other nutrients. This is especially important when soil P levels are already high. (I will talk about P pollution next time.)
From compost
Compost, typically having about 1 percent N, provides some N to the soil but not much. Well finished compost is a slow release form of N that helps build the reservoir of N as part of the soil OM. Roughly 5 to 15 percent of the total N in a finished compost will be available to the crop the year of application, so compost is best used for its soil building properties and not to supply N during the cropping year. However, if compost has been added annually, the applications add up and the slow release from the accumulated OM will probably supply all the N your crops need. Keep this in mind and do not continue to add compost to a soil high in OM from previous years of compost applications, because excess nutrients can leave the farm or garden and become pollutants.
From purchased organic nitrogen fertilizers
Seed meals such as soybean meal (6-1-1) are preferred sources of N for organic growers because they are the best dollar value for N except for manure. Their N is moderately available (faster than from fish meal or bone meal, slower than from blood meal). There is no risk of burning plants.
Alfalfa meal (2-1-2), a plant-based nitrogen source, releases N moderately fast. Its good C:N ratio is easy on the soil and it contains plenty of other nutrients in good balance, so this may be the best buy for many farms.
Fish Meal (9-3-0) is an excellent source of slowly to moderately released N when no potassium is needed. The high level of N is tied up in proteins, so there’s no risk of burning plants. The fish odor may attract animals.
Steamed bone meal (5-12-0, with 22 percent calcium) is an immediately available source of P that is good for side dressing early in the season, before biological activity gets going, but the N is tied up in very complex molecules and is very slowly released. Steamed bone meal has more N than precipitated bone meal, a common feed additive.
Chilean nitrate (16-0-0) is sodium nitrate, a naturally mined chemical with immediately available nitrate. It has no OM, and its N is soluble, so this material does not build soil. Thus, some people believe it does not meet the principles of organic farming. It may, however, be a good choice for a farmer who needs immediately available N with no other nutrients. Remember, under organic standards, only 20 percent of a crop’s N need may be met with Chilean nitrate.
So, how much nitrogen do you need to add? Calculate the amount with a pencil and paper. Many tables of N recommendations exist for growing particular crops (New England Vegetable Management Guide, Knott’s Handbook for Vegetable Growers, etc.), but you must calculate how much N your soil already has. Remember to credit previous crops, manures, compost and OM, then compare this total with the recommended value to see whether you need to add more.
Eric Sideman is MOFGA’s organic crops specialist. You can address your questions to him at 568-4142 or [email protected].

